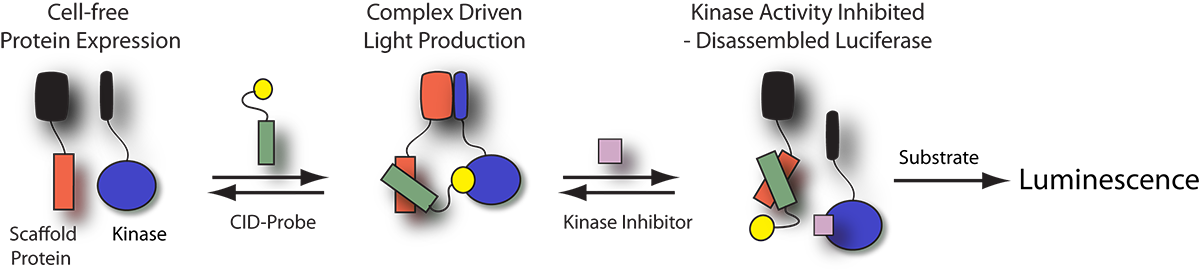
Kinase Profiling Panels
Applications:
- Rapidly screen test compounds or compound libraries at single concentration
- Quantitate inhibition in dose response titrations, measure IC50s
- Measure selectivity of an inhibitor against a kinase profiling panel
- Measure the impact of full-length vs kinase domain constructs on test compounds
Cell-free KinaseSeeker™ Assays
Luceome offers two assay formats for evaluating kinase inhibitors. The Cell-free KinaseSeeker assay uses the proprietary three-hybrid split-luciferase technology, where the two luciferase fragment-fusion proteins are expressed in rabbit reticulocyte lysate and the reassembly of split-luciferase, mediated by a CID-probe, takes place in this cell-free system. Competitive displacement of the CID-probe by a kinase inhibitor is measured by a change in luminescence signal. The assay is a homogeneous binding assay, which enables rapid kinase profiling in a high-throughput format.
Increasing examples have shown that regulatory domains outside of the kinase domain can greatly influence the activation state of the kinase and consequently impact inhibitor binding. The KinaseSeeker™ assay platform is highly amenable to full-length/intracellular domain testing. These full-length and intracellular-domain containing kinases provide for numerous opportunities to investigate autoinhibited states, conformation-selective and allosteric inhibitors that otherwise would not be available in a standard kinase panel. Luceome has over 90 such constructs (across multiple kinase families) available for profiling in the cell-free format.
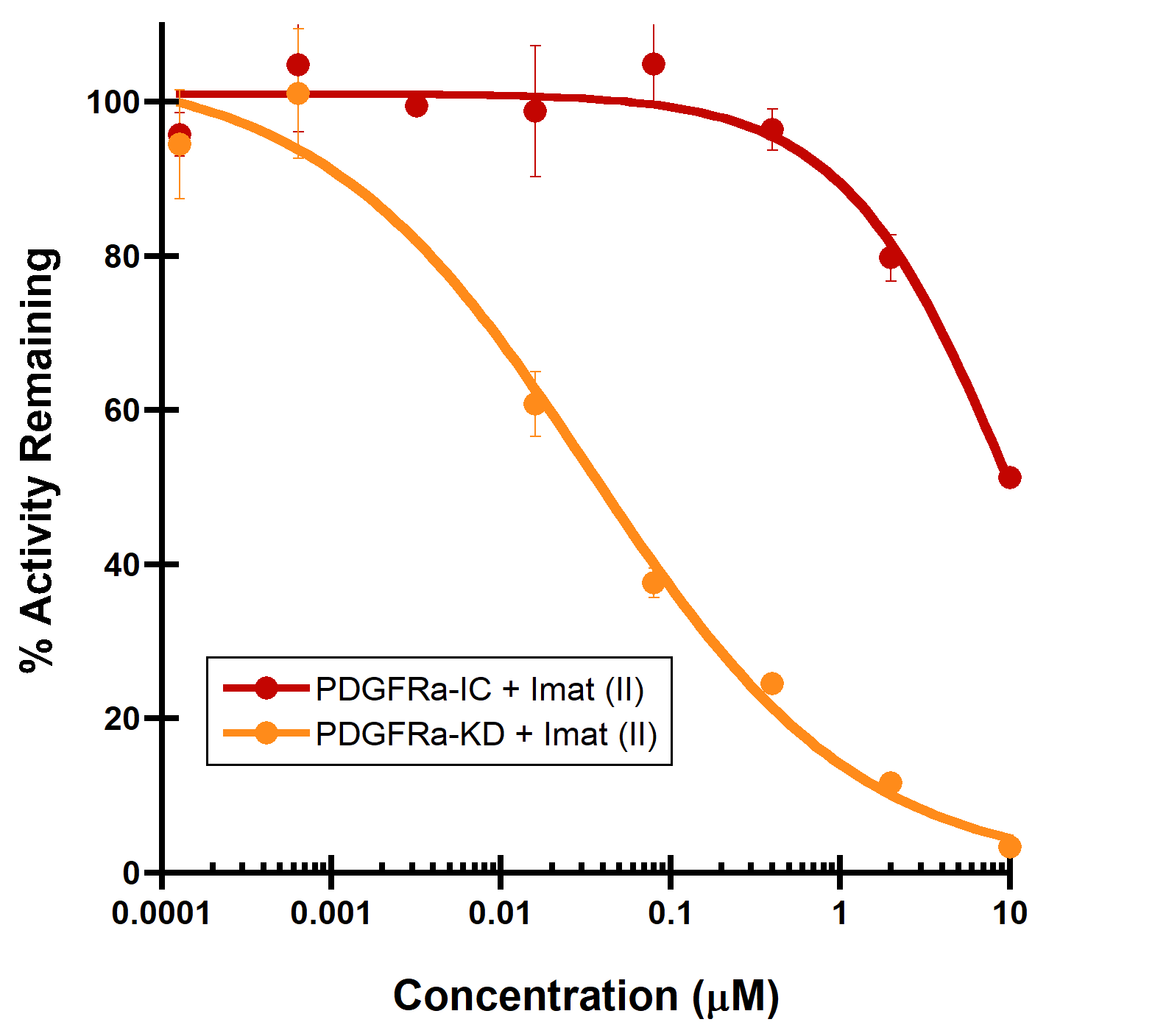
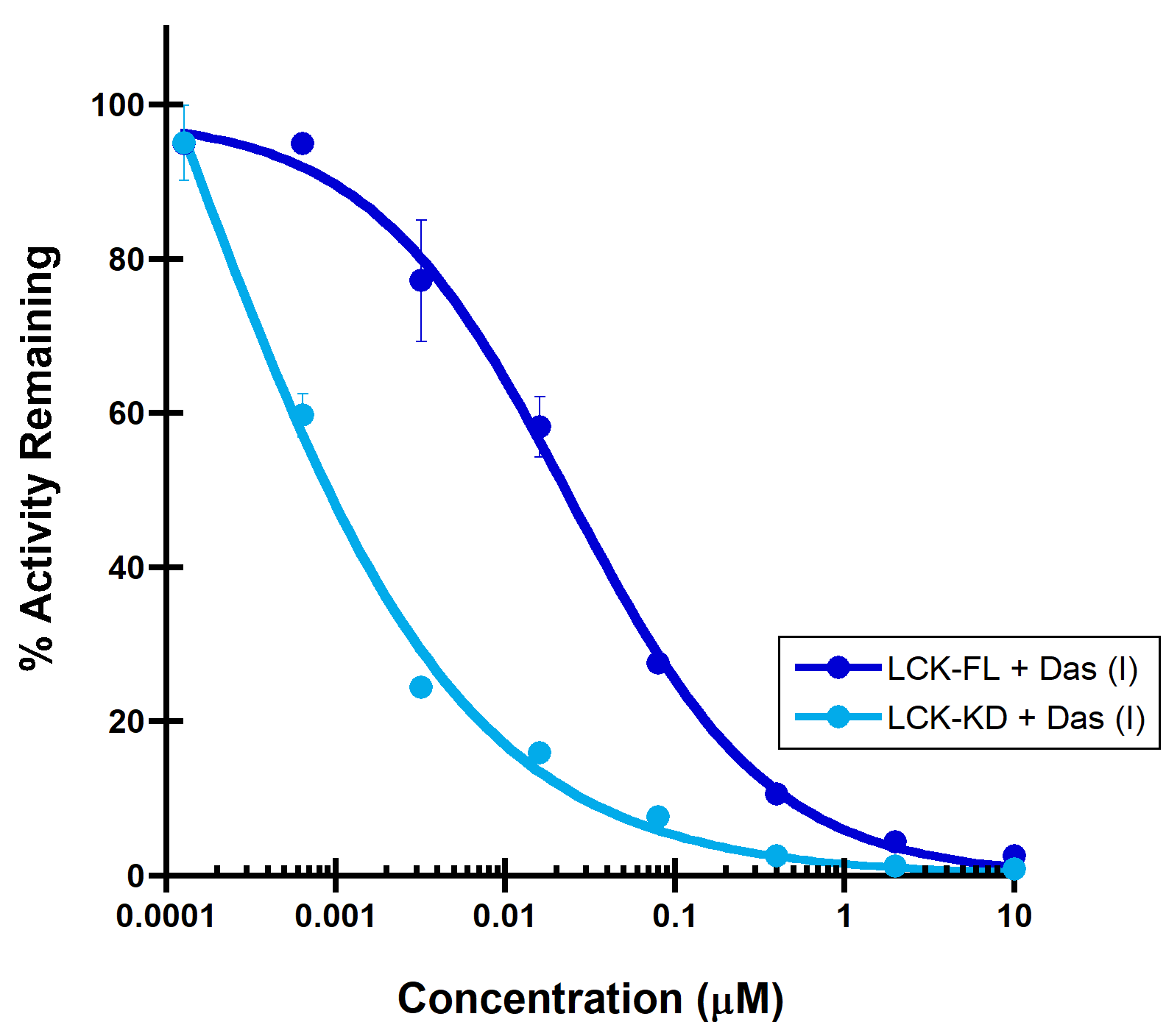
(A) PDGFRa binding to a Type II inhibitor. Type II inhibitor binds the autoinhibited, juxtamembrane containing PDGFRa-intracellular module with reduced affinity (IC50 > 10 µM), compared to the PDGFRa-kinase domain (IC50 = 44 nM). (B) LCK binding to a Type I inhibitor. A dramatic increase in binding potency is observed with the LCK-kinase domain (IC50 = 0.14 nM) over the SH2-SH3 domain containing LCK-full length construct (IC50 = 32 nM).